EverExceed เพิ่งเปิดตัวเทคโนโลยีใหม่ที่อุณหภูมิต่ำ
แบตเตอรี่ลิเธียมเหล็กฟอสเฟต
ซึ่งสามารถชาร์จได้แม้ในอุณหภูมิต่ำกว่า 0°C และอุณหภูมิติดลบ ในบทความทางเทคนิค 5 บทความ เราจะอธิบายรายละเอียดของเทคโนโลยีอันล้ำสมัยนี้อย่างละเอียด บทความนี้จะพูดถึง "การปรับปรุงวัสดุ" ของแบตเตอรี่ลิเธียมเทคโนโลยีอุณหภูมิต่ำ
การปรับปรุงวัสดุ:
การแนะนำ:
ในสองบทความแรก เราได้พูดถึงเรื่อง "ประสิทธิภาพ" และ "หลักการ" กันไปแล้ว ในบทความนี้ เราจะมาพูดถึงวิธีการปรับปรุงประสิทธิภาพที่อุณหภูมิต่ำกัน
ก่อนที่จะกล่าวถึงการปรับปรุงวัสดุ เรามาทบทวนกระบวนการชาร์จแบตเตอรี่ลิเธียมไอออนกันก่อน ซึ่งแบ่งออกเป็น 4 ขั้นตอน:
1) ไอออนลิเธียมจะถูกแยกออกจากอนุภาคบวกและเข้าสู่อิเล็กโทรไลต์
2) การถ่ายโอนลิเธียมไอออนในอิเล็กโทรไลต์
3) ลิเธียมไอออนสัมผัสกับขั้วลบผ่านฟิล์ม SEI
4) การแทรกและการแพร่กระจายของไอออนลิเธียมในอิเล็กโทรดลบ
เนื้อหาการปรับปรุงที่สำคัญที่จะกล่าวถึงต่อไปในบทความนี้ยังขยายออกไปทีละประเด็นจากสี่ประเด็นข้างต้นด้วย
——ไอออนลิเธียมจะถูกแยกออกจากอนุภาคบวกและเข้าสู่อิเล็กโทรไลต์
นี่คือจุดเริ่มต้นของการเคลื่อนที่ของลิเธียมไอออนในกระบวนการชาร์จ และเป็นขั้นตอนที่ง่ายที่สุดและมีความต้านทานน้อยที่สุดในบรรดาสี่ขั้นตอน ความต้านทานของลิเธียมไอออนแบบแคโทดอินเตอร์คาเลชันส่วนใหญ่ขึ้นอยู่กับโครงสร้างของวัสดุแคโทด ลิเธียมโคบอลเตตมีโครงสร้างแบบชั้น และสามารถฝังลิเธียมไอออนได้อย่างอิสระจากด้านหน้า ด้านหลัง ด้านซ้าย และด้านขวา ดังนั้นจึงมีประสิทธิภาพที่ดีแม้ในอุณหภูมิต่ำ โครงสร้างโมเลกุลของลิเธียมโคบอลเตตแสดงไว้ดังนี้
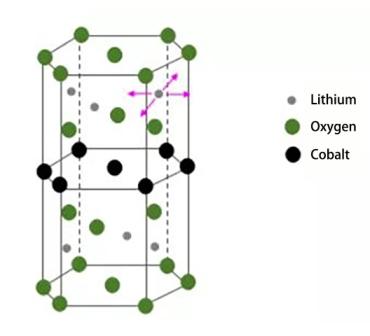
เมื่อเทียบกับลิเธียมโคบอลต์ออกไซด์แบบชั้น ลิเธียมเหล็กฟอสเฟตมีโครงสร้างโอลิวีน ในโครงสร้างนี้ PO4 จำกัดการเปลี่ยนแปลงปริมาตรของโครงสร้างผลึก ดังนั้นอิมพีแดนซ์ของการแทรกซึมและการสลายการแทรกซึมของลิเธียมไอออนจึงสูงกว่า และประสิทธิภาพสัมพัทธ์ที่อุณหภูมิต่ำยังไม่ดีเท่าลิเธียมโคบอลต์ออกไซด์
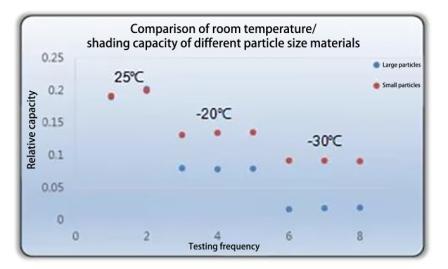
นอกจากนี้ สำหรับอนุภาคของวัสดุแอคทีฟ ยิ่งอนุภาคมีขนาดเล็ก เส้นทางการเคลื่อนที่ของลิเธียมไอออนก็จะสั้นลง ที่อุณหภูมิห้อง เนื่องจากการแพร่กระจายอย่างรวดเร็วของลิเธียมไอออน อิทธิพลของอนุภาคขนาดใหญ่และขนาดเล็กที่มีต่อความจุจึงยังไม่ชัดเจน แต่ที่อุณหภูมิต่ำ ข้อดีของวัสดุอนุภาคขนาดเล็กจะเริ่มปรากฏให้เห็น ผลการเปรียบเทียบความจุของอนุภาคของวัสดุชนิดเดียวกันที่อุณหภูมิต่างกันมีดังนี้
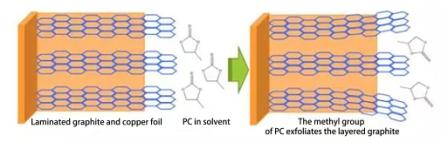
ลิเธียมไอออนจะถูกกำจัดออกจากแคโทดโดยมีการขัดขวางน้อยที่สุด และเข้าสู่อิเล็กโทรไลต์ได้อย่างมีประสิทธิภาพ ในอิเล็กโทรไลต์ ระดับการขัดขวางขึ้นอยู่กับค่าการนำไฟฟ้าของอิเล็กโทรไลต์ที่อุณหภูมิต่ำ เพื่อให้อิเล็กโทรไลต์มีประสิทธิภาพที่อุณหภูมิต่ำ จำเป็นต้องลดปริมาณตัวทำละลาย EC ที่มีจุดหลอมเหลวสูง (จุดหลอมเหลว 39-40 องศาเซลเซียส) โดยทั่วไปประมาณ 15-25% สามารถเติม PC ที่มีจุดหลอมเหลวต่ำ (จุดหลอมเหลว - 48.8 องศาเซลเซียส) ได้ แต่ควรเติมสารเติมแต่งที่ทำให้เกิดฟิล์มควบคู่ไปด้วย เพื่อป้องกันการลอกของชั้นกราไฟต์ที่เกิดจาก PC แผนภาพวงจรมีดังนี้:
ค่าการนำไอออนสูงเป็นค่ามาตรฐานของอิเล็กโทรไลต์อุณหภูมิต่ำ แต่ค่าการนำไอออนสูงที่อุณหภูมิห้องไม่ได้หมายความว่าจะมีประสิทธิภาพการทำงานที่อุณหภูมิต่ำที่ดีกว่าเสมอไป กุญแจสำคัญของปัญหานี้คือการรักษาค่าการนำไอออนที่อุณหภูมิต่ำ ค่าการนำไอออนถูกกำหนดโดยค่าคงที่ไดอิเล็กทริกและความหนืด ค่าคงที่ไดอิเล็กทริกหมายถึงปริมาณของ Li + ในสถานะอิสระที่ความเข้มข้นของเกลือลิเธียมเท่ากัน โดยธรรมชาติแล้ว ยิ่งมากยิ่งดี ความหนืดหมายถึงความต้านทานต่อการถ่ายโอน Li + โดยธรรมชาติแล้ว ยิ่งความต้านทานน้อยก็ยิ่งดี
บทสรุป:
เพื่อให้ตรงกับความต้องการของประเทศหนาวเย็นที่คุณต้องการความน่าเชื่อถือ
โซลูชันการจัดเก็บพลังงาน
สำหรับการใช้งานกลางแจ้ง วิศวกรวิจัยและพัฒนาของ EverExceed ได้ทุ่มเทเวลาอันยาวนานเพื่อคิดค้นโซลูชันที่เหมาะสม จึงทำให้เกิดเทคโนโลยีใหม่นี้ขึ้น ดังนั้น สำหรับโซลูชันการจัดเก็บพลังงานสำหรับอุณหภูมิเย็นของคุณ เลือกใช้ EverExceed เป็นแบรนด์ที่เชื่อถือได้อย่างสมบูรณ์



